Welcome To
Department of Pharmacology RMU
Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.Introduction of the department and message from HOD.
Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.Message from HOD.
Name
DesignationDepartment of Pharmacology
Department of Pharmacology
RMU
Report 2021 Dr. Asma Khan Chairperson CTU
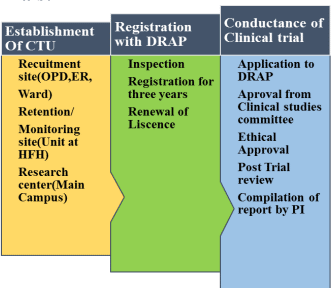
Importance of continuous research for clinical care and health education needs no more emphasis. Thus education, clinical care and research are the cornerstones for teaching health centres. In our country with limited resources, it is not always possible to execute research in its true meaning and essence. Limited resources and reduced funding had led the institutions to explore and develop linkage with the sponsors/industry and get it regularized with regulatory bodies of the country. In Pakistan DRAP (Drug regulatory authority of Pakistan) had started registering the institutes as Clinical trial Units after scrutinizing the facilities and infrastructure of the site. Clinical Trial Units (CTUs) have been set up with a purpose to design, conduct, analyse and publish clinical trials and other well-designed studies which can be monitored at any stage by the regulatory bodies.
Registration of Clinical trial site with DRAP:
Rawalpindi medical university has the mission to impart evidence based research oriented medical education and to provide best possible patient care. Under the guidance of worthy Vice Chancellor Professor Dr. Muhammad Umar, Rawalpindi medical University is licensed to act as Clinical Trial Site at Infectious Disease Department/ Department of Medicine in June 2021 to play its role in genuine research and establish linkage between industry and academia. Completion of the registration formalities are credited to the tireless efforts of Associate Professor Dr. Muhammad Mujeeb Khan (Chairperson Department of Infectious Diseases, RMU).


This clinical trial unit has the objectives of
• To conduct safe and efficient clinical trials for new treatment interventions.
• To compare the existing treatment options for more effective use and betterment of the patients.
• To explore alternative treatment strategies when the existing ones are not effective.
• To provide newer and expensive treatment options to the patients either free of cost or with minimal charges.
Road Map To Conduct Clinical Trials:
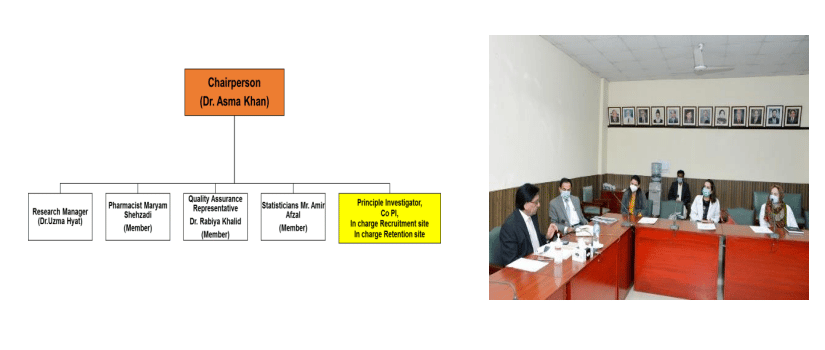
Clinical Trial Unit Team
To achieve the above goals and objectives, under the directives of Vice chancellor, Clinical trial unit Committee was formulated that started working enthusiastically from August 2021 onwards. For conduction of safe and efficient trials, Vice chancellor Professor Dr. Muhammad Umar had also allocated an amount of Rs 24.0 Million to establish CTU retention site, funded from HEC. Since its formulation, CTU team is continuously working to actualize the vision of a fully functional CTU. The Aim of CTU team is to develop the culture of conducting clinical trials in Rawalpindi Medical University that can meet the standards of clinical trials conducted in any advanced country. Rawalpindi Medical University is also registered with MedDRA to report any adverse events. In the period of mere 4 months, CTU team including Researchers, Principal Investigators and Sponsors are able to start three clinical trials, out of which two are registered with DRAP and NIH (USA).The details of these trials are:
Aims & Objectives
- -----------------------------------------
- -----------------------------------------
- -----------------------------------------
- -----------------------------------------
- -----------------------------------------
- -----------------------------------------
- -----------------------------------------
- -----------------------------------------
Publications
Sr.
Title Of Trial
Status
1
Efficacy and Safety of Apixaban in Covid-19 Coagulopathy patients with Respiratory Severity Under Critical care (ECRU TRIAL)
Registered https://clinicaltrials.gov/ct2/show/NCT05088928
2
Vorexetine is a novel Anti-depressant with improvement in cognitive Abilities(VENUS)
Registered https://clinicaltrials.gov/ct2/show/NCT05104918
3
Loading dose of Vitamin D under comparison through two different Oral delivery system(PLUTO)
In process
4
Evaluation of a new life style change for the management of GERD
In process
5
To establish the efficacy of Tofojak in patients with Ulcerative Colitis
In process
Under the patronage of Vice Chancellor, entire CTU team endeavours to put in focused efforts
for achieving efficient and better treatment options through authentic research in medicine.